written by Michael Himmelbauer
Firstly, you put a few grams of two white powders into a beaker and after stirring for a minute, it turns into a turbid liquid. What sounds to be impossible (or fake news, as we would call it), can be realized with the help of some components you may not have at home, but luckily can be found in the chemistry room:
- two beakers
- a spoon
- a scale
- a brick of wood
- the stars of the experiment: 15 grams of Barium hydroxide (Ba(OH)2) and 5 grams of Ammonium thiocyanate (NH4SCN)
- some water (H2O)

First of all, we weigh the required amount of the two powders with the help of the scale and put them into the two beakers as shown in the picture:

Next, we do something you better should not do if you don’t know what’s inside the beakers (fortunately, I know what I’m writing about): We pool the two powders into one beaker (in case you’ve got two different sizes: put it into the bigger one) and mix them with the help of the spoon. After some time stirring (and maybe a hurting arm), we recognize that inside the beaker, the powders disappeared and were replaced by a turbid white liquid. Furthermore, the fluid smells strange. In case you touch the bottom of the glass, you may find out that it feels cold (although the windows and the fridge are closed, so the cause of the low temperature is the beaker – even if you don’t believe it).

But why’s that? How can two powders that look almost similar turn into a liquid that stinks and cools down its environment?
To explain that chemical process, we have to take a look at the reaction equation of Barium hydroxide with Ammonium thiocyanate:
Ba(OH)2 + 8 H2O + 2 NH4SCN → Ba(SCN)2 + 2NH3(g) + 10 H2O
According to the equation, the educts Barium hydroxide (that consists of Ba(OH)2 and solid water (H2O)) and Ammonium thiocyanate (NH4SCN) react with each other. The products on the right side of the equation are Barium thiocyanate (Ba(SCN)2), Ammoniac (NH3) and liquid water (H2O). The turbid liquid consists of Barium thiocyanate and water, Ammoniac is released into the air as a gas that causes the unpleasant smell.
And in case you put the beaker onto the wept brick of wood, you might be frightened when you want to lift the glass because you may recognize that the ice-cold beaker sticks onto the brick of wood:

But why can a liquid that consists of two strange powders make a glass stick on a wooden part?
In order to answer this question, we have to know that the chemical reaction described above is considered to be an endotherm reaction as energy (in the form of warmth) is required all the time. This energy is taken from the environment around the powder in the glass, and that is why the glass cools down. And as we all know (or have at least learnt at school), water freezes in case the temperature drops below zero degrees. Caused by the fact that we put some water onto the brick of wood before mixing the powders, the breaker has frozen onto it.
The energy graph (a diagram that shows the amount of energy a substance contains) of an endotherm chemical reaction outlines that the products in the end have a higher level of energy than the educts at the beginning. In order to compensate the difference, energy in the form of heat is required and is taken from the environment.
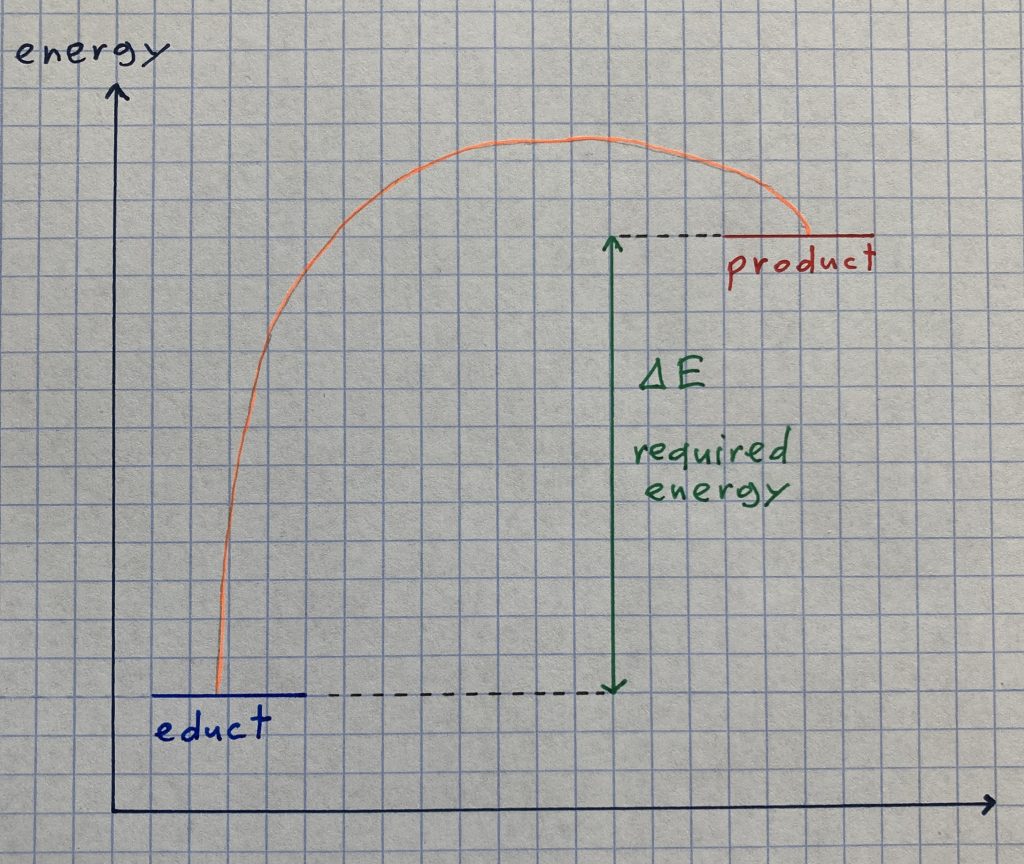
To sum up, it can be stated that when pooling two special powders into one beaker, their state of aggregation turns into liquid and the required energy can make the glass freeze onto a brick of wood. Both are results that wouldn’t come to your mind when you’re thinking about powders, would they?
source:
Anon.: Entropische Zauberei. https://www.uni-wuerzburg.de/fileadmin/08020000/pdf/erlebnis/endotherm_reak.pdf
[last access: 01.02.2022]
fotocredit: (c) by Michael Himmelbauer
